Listen to the Podcast
Background & Initial Management
Like any sick child the initial management of the ‘Collapsed Neonate’ involves an ABCDE approach. While there are many possible causes, four major diagnoses must be considered:
- Sepsis – Explore history for risk factors for sepsis e.g. maternal group B streptococcus (GBS) colonisation or a sibling affected with GBS sepsis, prolonged rupture of membranes > 24 hours prior to delivery or evidence of chorioamnionitis (maternal pyrexia, foul smelling discharge, uterine tenderness or elevated maternal inflammatory markers). Neonates with sepsis often present with non-specific symptoms such as lethargy, vomiting, poor feeding, temperature instability (high or low), apnoea and signs of shock (skin mottling, tachycardia, prolonged capillary refill time, elevated lactate and reduced level of consciousness). The absence of an inflammatory marker rise or the lack of risk factors does not exclude sepsis and ALL neonates presenting in a collapsed state must be treated with antimicrobials pending culture results. Intravenous Cefotaxime and Amoxicillin (both 50 mg/kg) are commonly used as combined they provide good coverage against possible organisms (GBS, E Coli and Listeria). Disseminated herpes simplex presents with similar features to bacterial sepsis and consideration should be given to empirically adding intravenous Aciclovir (20 mg/kg) to the above antibiotics (particularly if the baby has a vesicular rash, liver disfunction or coagulopathy). See the Sepsis Chapter for further details
- Congenital Heart Disease – Enquire about whether the antenatal scans were normal, was there any concerns on the neonatal discharge examination and is there any family history of congenital heart disease. The ductus arteriosus connects the pulmonary artery to the descending aorta and in utero it allows blood to bypass the fluid filled lungs. It normally closes in the first few days after birth, however while it remains patent neonates with serious cardiac problems may appear stable as the duct provides a source of either systemic blood (obstructive left heart lesions e.g. hypoplastic left heart syndrome, coarctation of the aorta, interrupted aortic arch or critical aortic stenosis), pulmonary blood (obstructive right heart lesions e.g. tetralogy of fallot, critical pulmonary stenosis, tricuspid atresia or pulmonary atresia) or mixing (transposition of the great arteries). It is only once the duct starts to close that the baby becomes unwell. The obstructive left heart lesions present with a combination of signs of shock and congestive heart failure when the duct closes. The obstructive right heart lesions and transposition of the great arteries present with increasing cyanosis when the duct closes. Suspect congenital heart disease for hypoxia refractory to administration of oxygen, hypoxia without significant respiratory distress/lung pathology on chest radiograph, pathological murmur, absent femoral pulses, cardiomegaly on chest radiograph, hepatomegaly or lactic acidosis refractory to intubation/cardiovascular support. Specific management involves urgently starting a prostaglandin infusion in an attempt to reopen the ductus arteriosus, echocardiogram (if local skills allow) and discussion with a paediatric cardiologist (conference call via retrieval team). NB certain cardiac lesions may be time critical requiring urgent transfer to a cardiac centre e.g. transposition of the great arteries needing urgent atrial septostomy or obstructed total anomalous pulmonary venous drainage which will be refractory to conservative management (prostaglandin can make things worse) and needs urgent surgical repair.
- Metabolic – Enquire about a family history of metabolic problems or sudden infant deaths and check for consanguinity of parents. Suspect if multi-system disorders presenting with lethargy, abnormal tone, seizures, hepatomegaly, shock, metabolic acidosis or hypoglycaemia. Many of the tests required as part of the neonatal metabolic screen will take several days to come back, however an ammonia must be processed urgently (result should be available in most laboratories within an hour) and as ammonia is toxic to the brain, if found to be elevated reducing it must be considered to be time critical and discussion with the metabolic team (conference call via retrieval team) should be arranged. Further information is included later in the chapter on investigations to send and how to make up the infusions required for treatment of hyperammonaemia.
- Non-Accidental Injury – Suspect if obvious injuries, inconsistent history or delayed presentation (but should be considered in all patients). Neonates have a total blood volume of approximately 80 ml/kg (320 ml in a 4kg baby) and therefore can easily become shocked from an intracranial bleed. After initial resuscitation, consider urgent neuroimaging in the baby presenting with unexplained reduced consciousness or seizures and a bulging fontanelle. Haemorrhage disease of newborn can present with spontaneous intracranial bleeding and history of postnatal vitamin K administration should be sought.
Other diagnoses to consider include tension pneumothorax, pericardial effusion, myocarditis, arrhythmia (sinus tachycardia rarely exceeds 220 bpm in neonates), intussusception/volvulus, toxin ingestion, anaphylaxis, adrenal insufficiency (shock together with hyperkalaemia, hyponatraemia and hypoglycaemia) or hypothyroidism. Neonates with bronchiolitis who develop respiratory failure, apnoea or airway obstruction in the community often present in a collapsed state, however they normal respond quickly to resuscitation.
Airway
Electively intubate if using a prostaglandin infusion > 15 nanogram/kg/min and external transfer is required, or if a ‘high dose’ (> 20 nanogram/kg/min) prostaglandin strategy is planned. Intubation should also be considered for neuroprotection i.e. intracranial haemorrhage (NAI/haemorrhage disease of the newborn) or for cerebral oedema (meningitis/metabolic problem) or to help balance the circulations in pulmonary overcirculation e.g. hypoplastic left heart syndrome with high saturations (>85%) and signs of inadequate systemic circulation (elevated lactate, poor perfusion). Other indications for intubation include respiratory failure, recurrent apnoea, airway obstruction/loss of protective airway reflexes, GCS < 8, to facilitate safe imaging/line insertion or ongoing signs of shock unresponsive to 40 ml/kg of fluid resuscitation. NB Cardiovascular resuscitation should occur before induction of anaesthesia in the shocked patient.
Support patient with PEEP using Ayre’s T-Piece and face mask while preparation for intubation are made. Preoxygenate using an oxygen concentration suitable for patient’s suspected pathophysiology i.e. use 100% oxygen for suspected sepsis, metabolic problem or raised ICP and try to limit oxygen to 30-40% in suspected duct dependant congenital heart disease (excessive oxygen may cause the duct to close or cause pulmonary overcirculation/systemic collapse in a baby with hypoplastic left heart syndrome).
If cardiovascular stability has not been established following fluid resuscitation start peripheral/intraosseous adrenaline at 0.1 mcg/kg/min (1 mg of adrenaline in 50 ml of 0.9% saline infused at 0.3 x weight in kg ml/hr = 0.1 mcg/kg/min) and titrate to effect PRIOR TO ADMINISTRATION OF INDUCTION AGENTS (remember to ensure adrenaline has reached patient through the line dead-space). If cardiovascularly stable rather than starting an infusion prepare ‘Push Dose’ adrenaline (1 in 100,000) by diluting 1 ml of adrenaline 1 in 10,000 with 9 ml of 0.9% saline to be used to treat any cardiovascular instability on induction (dose is 0.1 ml/kg repeated as required).
The collapsed cardiac or septic neonate is unlikely to tolerate intubation well. Brief all team members of the high risk of cardiac arrest on induction and have resuscitation drugs prepared. In the absence of an arterial line ensure the non-invasive blood pressure is cycling every minute and allocate a member of staff to keep a finger on the pulse.
Insert a nasogastric tube and aspirate stomach prior to induction and task someone to continue to aspirate air from the stomach as mask ventilation commences (gentle ventilatory support during the apnoea period will be required and despite this desaturation is still likely, even with slick intubation).
Administer an additional 10 ml/kg bolus of 0.9% saline (in addition to resuscitation fluid already given immediately prior to induction). There is a high risk of vagally mediated bradycardia in the sick neonate, so administer 20 mcg/kg of atropine prophylactically.
Propofol, thiopentone and midazolam should not be used for induction in the haemodynamically unstable neonate. Although ketamine is the safest induction agent, due to exhaustion of endogenous catecholamines, cardiovascular instability should still be expected with its use in this situation. Modified RSI with ketamine (reduce dose to 0.5 – 1 mg/kg) and rocuronium 1 mg/kg by most experienced operator. Expect the effects to take longer than usual to become evident due to impaired circulation time and avoid the temptation to give more too quickly.
During intubation keep head in neutral position and have suction to hand (secretions can easily block view of cords in small infants).
A straight blade generally provides a better view than a curved blade in neonates due to laxity in the glossoepiglottic ligament (pressure is the vallecula with a curved blade is likely to result in inadequate elevation of the epiglottis and a suboptimal view). You should be able to intubate all neonates using a Miller size 1 blade. This blade is designed to lift the epiglottis directly (video 1) rather than be inserted into the vallecula, however if lifting the epiglottis is proving difficult, it can still be inserted into the vallecula and when combined with bimanual laryngoscopy (external manipulation of the larynx by the intubator) a satisfactory view allowing intubation is normally obtained (video 2).
Use a 3.0mm cuffed endotracheal tube for term infants >3kg, a 3.0mm uncuffed tube for preterm neonates/weight < 2.5 kg and a 3.5mm uncuffed endotracheal tube for preterm neonates/weight ≥ 2.5 kg. Positioning the depth marker on the endotracheal tube at the level of the cords provides the best method of estimating the correct depth of insertion, however this depth can also be determined in advance by using the following formulae in neonates:
Oral ET tube length (cm) = weight (kg) + 6
Nasal ET tube length (cm) = weight (kg) + 7
Perform a chest radiograph to confirm endotracheal tube position.
Breathing
Routine settings on ventilator i.e I:E ratio 1:2, PEEP 6 cmH2O, Ti 0.6 seconds, rate 30 and adjust depending on blood gases.
A peak pressure of around 20 cmH2O or tidal volume of 6 ml/kg is a reasonable starting point and adjust depending of chest movement and blood gases.
Ensure the ventilator/circuit you are using is suitable for use in infants (this is a common cause refractory hypercapnia in this age group). Likewise use of large filters, oversized capnography or angle pieces all increase the dead-space and may cause hypercapnia.
Continuously monitor pulse oximetry (preductal on right hand and post ductal on either foot if duct dependant congenital heart disease) and capnography.
Target oxygen saturations depend on presumed pathophysiology. If oxygen delivery is important e.g. sepsis, raised intracranial pressure target saturations of 100%. In duct dependant congenital heart disease target saturations of 75 – 85%. In this scenario ideally ventilate in air, but FiO2 can be increased up to 0.3 – 0.4, however the addition of oxygen will not help if the hypoxic is due to a flow or mixing problem and may in fact make things worse if it prevents the duct from reopening (in this setting increasing the prostaglandin dose or septostomy may be required). Don’t be reassured by high saturations (>85%) in a single ventricular circulation as this indicates the systemic and pulmonary circulations are not balanced and there is excessive pulmonary blood flow at the expense of the systemic circulation (increasing of the pulmonary vascular resistance by reducing FiO2, hypoventilating, increase PEEP and reducing the systemic vascular resistance will help balance the circulation). Monitor lactate as a marker of the adequacy of systemic oxygen delivery.
Circulation
Consider using intraosseous access first-line for neonates needing immediate resuscitation with poorly visible veins (alternatively allow a maximum of two attempts or 90 seconds at peripheral venous access in this setting before moving on to intraosseous access). Scalp veins often offer the best chance of success initially and the external jugular vein should be considered once positioning head down with neck extended (place roll under shoulders) can be safely performed. Umbilical venous access can be attempted in neonates under one week old and a standard paediatric 5 Fr triple lumen central line can be placed in the umbilical vein with aseptic technique, however as intraosseous access is quicker it should be used in preference where immediate access is required.
If central access is required the femoral site is generally preferred using ultrasound guidance. Vasoactive drugs can be given initially via a peripheral or intraosseous line while central access is being obtained, so if you are not comfortable with inserting central lines in neonates discuss with retrieval team whether the central line really needs to be inserted prior to retrieval teams arrival. If the cardiac diagnosis is known avoid neck lines in babies who will need a Fontan procedure at some stage e.g. hypoplastic left heart syndrome and avoid femoral vessels in babies who may need a septostomy performed e.g. transposition of the great arteries (damage to the femoral veins may make performing this procedure impossible).
Arterial access should ideally be obtained in a peripheral artery e.g. radial or posterior tibial artery using a 24G cannula (using larger cannula in this age group increases risk of limb ischaemia). If this is unsuccessful the femoral artery should be used in preference to the brachial artery due to the low risk of complications (both end arteries but larger size of femoral artery allows more blood flow around cannula). Ultrasound is very helpful in shocked babies with poorly palpable pulses (use transfixation technique if using ultrasound).
Minimal targets for blood pressure in a term neonate are 60/30 (MAP 40). For preterm neonates the minimal mean arterial pressure is their corrected gestational age in weeks e.g. 36 mmHg for a 36 week old neonate.
For signs of cardiovascular shock (tachycardia, hypotension, prolonged capillary refill time, elevated lactate and poor pulse volume) administer fluid boluses of 0.9% saline in 10 ml/kg aliquots. Central venous pressure, pulse pressure variation on the arterial line with respiration and liver pressure can all be used to help guide fluid resuscitation (see Sepsis Chapter for details).
If more than 40 ml/kg of fluid resuscitation are required use vasoactive drugs to support the cardiovascular system i.e. start peripheral/intraosseous adrenaline at 0.1 mcg/kg/min (1 mg of adrenaline in 50 ml of 0.9% saline infused at 0.3 x weight in kg ml/hr = 0.1 mcg/kg/min) and titrate to effect. See Sepsis Chapter for further advice on titrating vasoactive drugs. For fluid and inotrope resistant shock add intravenous hydrocortisone 2.5 mg/kg every 6 hours and see differential diagnosis in Sepsis Chapter.
Dinoprostone (prostaglandin E2) should be started for suspected duct dependant congenital heart disease e.g. hypoxia refractory to oxygen, hypoxia without significant respiratory distress or lung pathology on chest radiograph, pathological murmur, absent femoral pulses, cardiomegaly on chest radiograph or lactic acidosis refractory to intubation and ventilation. The starting dose of dinoprostone depends on what you are trying to achieve. To maintain patency of an open ductus arteriosus it is reasonable to start at 5 nanogram/kg/min (not applicable to this scenario). However in the collapsed neonate the duct has closed and you are trying to reopen it, therefore you need to start at a much higher dose e.g. 20 nanogram/kg/min and increase rapidly to 50 nanogram/kg/min if ineffective (this can be further increased up to a maximum of 100 nanogram/kg/min). At high doses of prostaglandin apnoea and hypotension (due to vasodilation) are common i.e. intubation and ventilation will be required for transfer if doses > 15 nanogram/kg/min are used and prepare noradrenaline to support blood pressure when using high dose prostaglandin.
Continuously monitor ECG. Checking 4-limb blood pressures maybe helpful in determining the diagnosis, however echocardiogram should be performed if skills to do so locally are available.
Disability
Sedate with morphine 10 – 30 mcg/kg/hr. Avoid the routine administration of midazolam (high risk of withdrawal in this age group).
Boluses of non-depolarising muscle relaxant can be given as required (avoiding continuous infusion allows assessment of neurology).
Treat seizures with 20 mg/kg of phenobarbitone over 20 minutes (avoid phenytoin in cardiovascularly unstable patients) and look for an underlying cause e.g. metabolic problem, electrolyte/glucose abnormality or intracranial pathology. While meningitis may be responsible for the seizures, a lumbar puncture is contraindicated and MUST NOT be performed at this stage.
Monitor blood sugar and assess pupillary reflexes regularly. If hypoglycaemic perform a hypoglycaemia screen (see Labs & Electrolytes) and administer 2 ml/kg of 10% dextrose.
Sepsis
Early appropriate antibiotics should be given as soon as possible and must be given within 1 hour of presentation to all neonates presenting in a collapsed state. Ideally take blood culture prior to the administration of antibiotics, provided this doesn’t significantly delay their administration. A lumbar puncture is contraindicated in the acute phase of septic shock and MUST NOT be performed.
For community acquired sepsis administer intravenous cefotaxime (50 mg/kg) and amoxicillin (50 mg/kg). For hospital acquired sepsis administer 40 mg/kg of meropenem +/- 15 mg/kg of vancomycin (indwelling central line or known MRSA).
Intravenous aciclovir (20 mg/kg) should also be added to cover congenital herpes simplex infection.
The uncovered neonate will get cold very quickly. Limit time exposed and use warming techniques as required.
Renal
Restrict intravenous fluids to 100 ml/kg/day (normal neonatal maintenance fluids 150 ml/kg/day).
Use isotonic fluids (risk of SIADH) e.g. 0.9% saline and 10% dextrose +/- added KCL.
Catheterise bladder and monitor urine output (aim ≥ 1ml/kg/hr).
Gastrointestinal
Keep nil by mouth.
Insert and aspirate nasogastric tube (to remove any swallowed air splinting the diaphragm), then leave on free drainage.
Labs & Electrolytes
Routine Tests – FBP, clotting, group and save, U&E, Ca, Mg, PO4, LFTs, glucose, CRP, Ammonia, blood gas, lactate, blood cultures and whole blood (EDTA) for meningococcal and HSV PCR.
Hypoglycaemia Screen – blood for lab glucose, lactate, 3-hydroxybutyrate (ketones), insulin, C-Peptide, cortisol, growth hormone, plasma amino acids and acetyl-carnitine profile. Urine for ketones and organic acids.
Metabolic Screen – Blood for plasma amino acids, lab glucose, lactate, ammonia and GAL-1-PUT. Urine for ketones, reducing substances, organic and amino acids and orotic acid.
Drugs & Infusions
Dinoprostone (Prostoglandin E2)
- Indication: Used to reopen ductus arteriosus in suspected duct dependant congenital heart disease.
- Dosing range: 5 – 100 nanogram/kg/min.
- Side effects: apnoea, pyrexia, flushing, vomiting and hypotension.
- Administration: Withdraw and discard 0.5 ml from a 500 ml bag of 5% dextrose or 0.9% saline and add 0.5 ml of Dinoprostone (1 mg/ml) to the remainder of the bag to make a solution of 1 mcg/ml. Withdraw 50 ml of the reconstituted solution and administer via a syringe pump (1.2 x weight in kg ml/hr = 20 nanogram/kg/min).
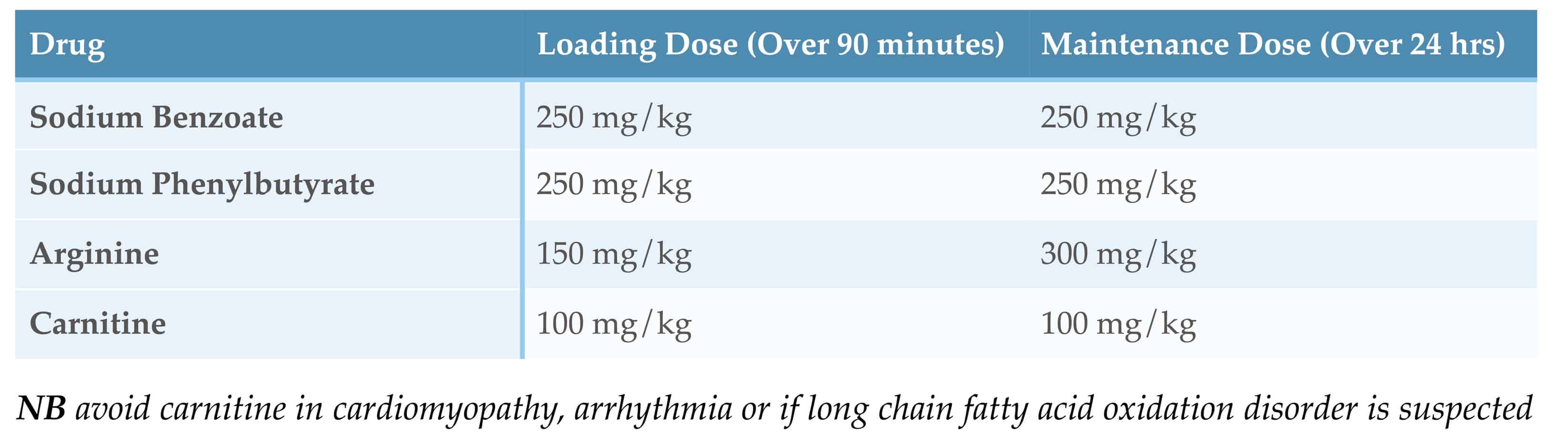
Drugs Used in Undiagnosed Hyperammonaemia
See the ‘British Inherited Metabolic Disease Group’ guideline on ‘Undiagnosed Hyperammonaemia Diagnosis and Immediate Management’ and their ‘Infusion Calculator for the Treatment of Hyperammonaemia’
![]()

Best Paediatric podcasts for Emergency Medicine, Critical Care and Prehospital and Retrieval Medicine. 5 star
Thanks Martie, I appreciate the feedback.
Excellent description on how to approach a collapsed neonate! Extremely helpful, cheers! (Registrar in Emergency medicine )
Medical student in Ireland studying for paediatric final – really helpful podcast, its very difficult to find such information all in one place and very clear description! Thank you I will certainly pass the recommendation on.
Glad you found it useful
Thank you, a really useful summary.
Glad you found it useful
Revising for final frca viva and found this very useful
Brilliant thank you! Can I ask what program you use to record your podcast? I’m trying to create some material for ED exam candidates and I’m struggling to download video from my iPhone
My go to for education on sick babies and children. The annual conference is great and very well executed. Thank you.
I have moved to an ED that has no functioning resuscitaire. I am planning to put forward a QIP that this is crucial equipment for neonatal resuscitation for newborn babies in ED.
My question is; do you think the resuscitaire is required equipment/provides better quality care in a non newborn baby i.e. the first 28 days of life who presents collapsed?
This will widen the use of the equipment and strengthen my argument.
Thanks in advance.